Tim Coker and Olivia Seifert
Earlier diagnosis can make all the difference for individuals with genetic diseases. Delayed diagnosis hinders timely disease management and treatment access, meaning that progression to late-stage disease can occur more rapidly, likely leading to a significant increase in the health and economic burden of these conditions for patients, their families, and healthcare systems. Screening programs aim to identify individuals with genetic conditions earlier, with cascade screening in particular providing one way to help transform outcomes for patients.
What is cascade screening?
Cascade screening is a systematic approach to genetic testing that is used to identify carriers of genetic mutations within families. The process begins when an individual is diagnosed with a genetic disorder – this individual is known as the “proband” – leading to their immediate family members, such as siblings, parents, and children, being tested for the same condition. In the event that these relatives are also found to carry the same mutation, the screening process is repeated within their own immediate family to identify additional disease carriers. This cascade effect helps identify asymptomatic carriers, enabling earlier diagnosis and intervention.
The benefits of cascade screening include:
Cascade screening is particularly effective for autosomal dominant diseases because approximately 50% of first-degree relatives of the proband would also be expected to be carriers. Screening is also applicable to autosomal recessive conditions, for which full siblings of the proband have a 25% chance of inheriting both mutated genes (and therefore being affected) and a 50% chance of being carriers.
Cascade screening can also significantly reduce the time to diagnosis for patients with rare genetic diseases, many of whom may otherwise endure a long ‘diagnostic odyssey’ – a quest for an accurate diagnosis that may last several years – as the rarity of many conditions means that many healthcare professionals may never have encountered the condition, or may not recognise the condition, so it goes undiagnosed or misdiagnosed for years.
An example of cascade screening success: Familial hypercholesterolaemia
Familial hypercholesterolaemia (FH) is an autosomal dominant disease that leads to increased cholesterol levels in the blood, increasing an individual’s chances of developing heart disease. FH is primarily linked to mutations in the LDLR gene, but it can also be caused by mutations in the APOB or PCSK9 genes.
A recent cost-effectiveness review of 21 studies evaluating 62 screening strategies for FH compared to the standard care for FH detection, found that cascade screening resulted in the largest health benefits per person tested.
Demonstrating the value of cascade screening using microsimulation modelling
Microsimulation-based modelling is a powerful technique for demonstrating how cascade screening can positively impact health and economic outcomes within a population. The modelling can quantify how many deaths, late-stage disease cases, adverse events, disease complications, years of working life, and direct and indirect healthcare costs would be avoided or saved, and the number of QALYs (quality-adjusted life years – with one QALY representing one year of life lived in perfect health) gained.
Microsimulation modelling is based on creating a “virtual population” that reflects a real-world population of interest. A key strength of microsimulation modelling is its ability to simulate dynamic, individual-level changes over time, capturing the complex interactions between individuals within a population. As a result, it allows for the health and economic outcomes under a cascade screening intervention to be projected into the future for a population of interest.
To project the impacts of a cascade screening intervention, a “family cohort structure” has been integrated into HealthLumen’s microsimulation model. This approach combines data on the genetic prevalence of a rare genetic disease with penetrance data (reflecting the proportion of individuals carrying a specific genetic variant who actually show symptoms of the associated condition) and mortality data from the real population of interest, to generate a virtual population of symptomatic individuals. Population data from the real population of interest, such as fertility rates and mean age at childbirth, are then input to create a realistic family cohort structure around these symptomatic individuals (Figure 1).
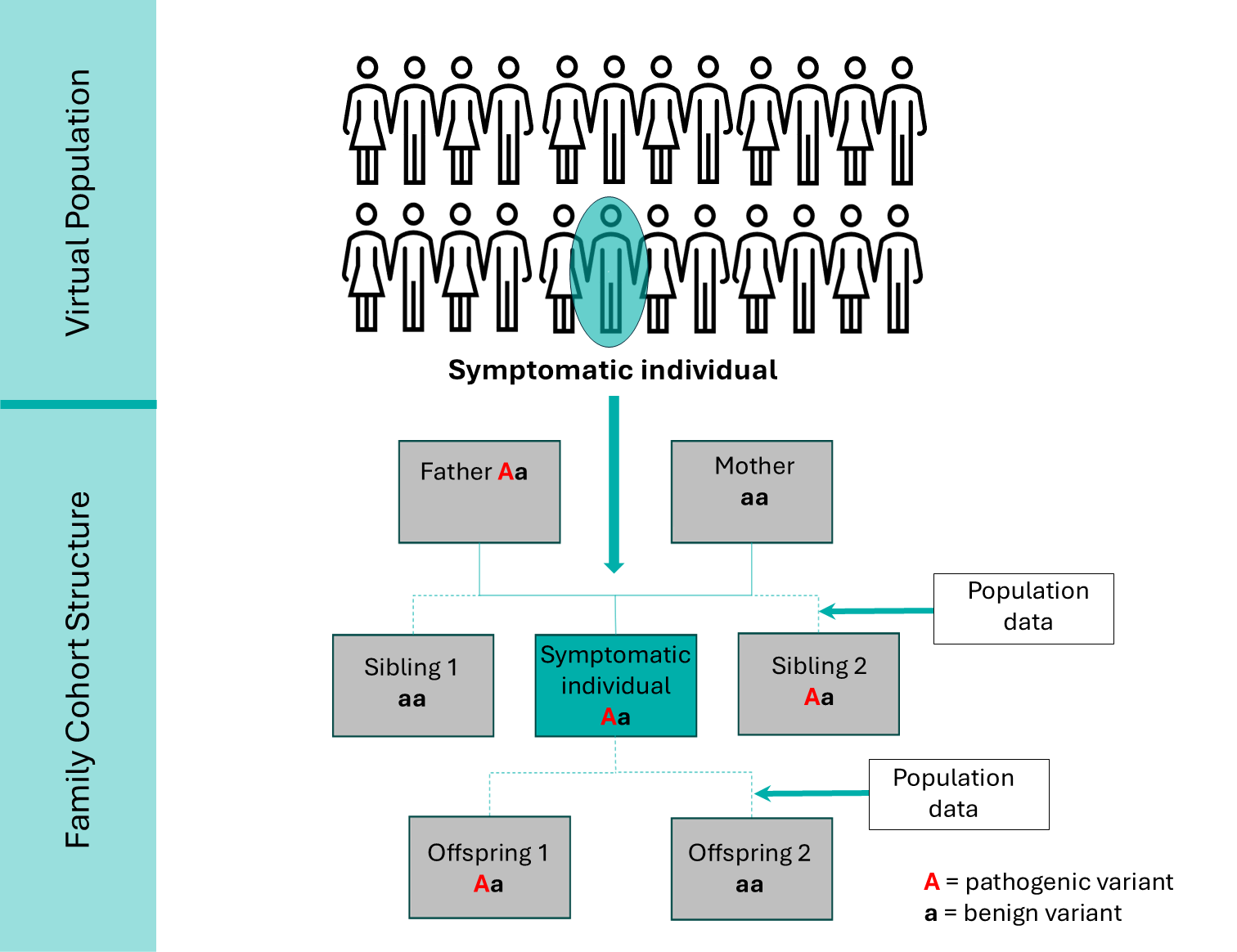
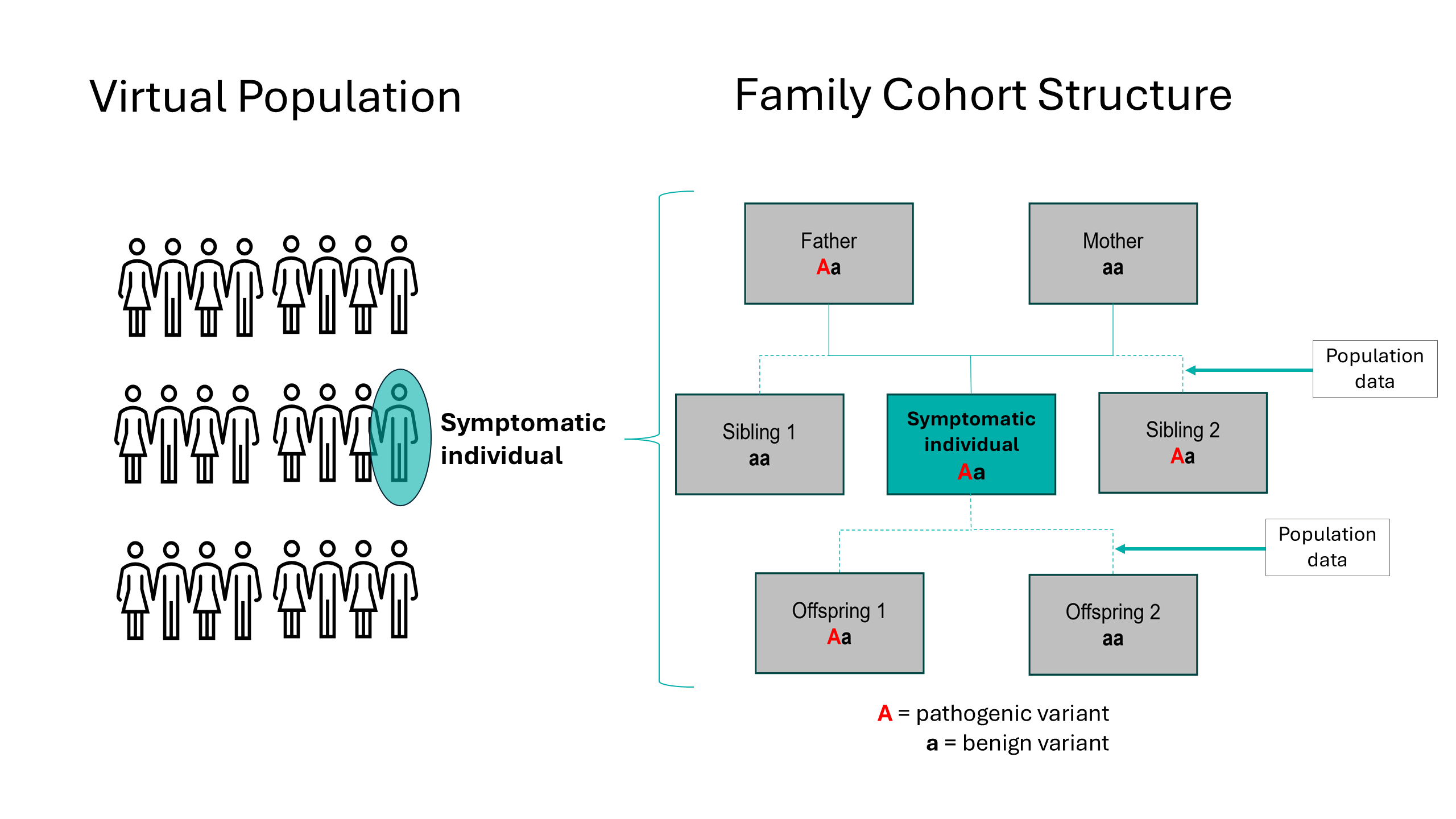
Figure 1: Family cohort structure constructed around a symptomatic individual for an autosomal dominant genetic condition, used for the microsimulation modelling of a cascade intervention.
The model assumes that once symptomatic individuals are diagnosed, their first-degree relatives are identified and recommended for genetic screening as part of a cascade screening process described in Figure 2.
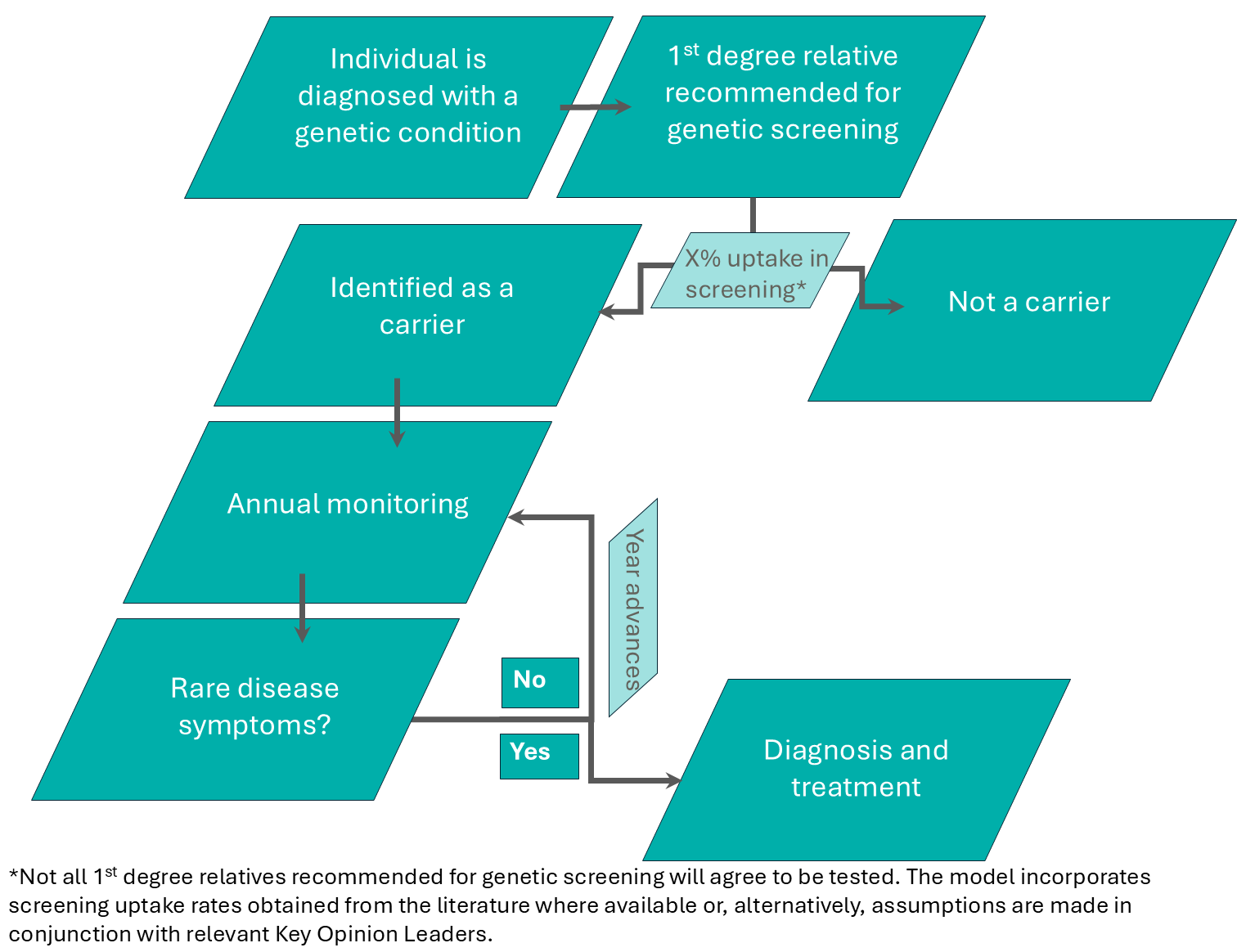
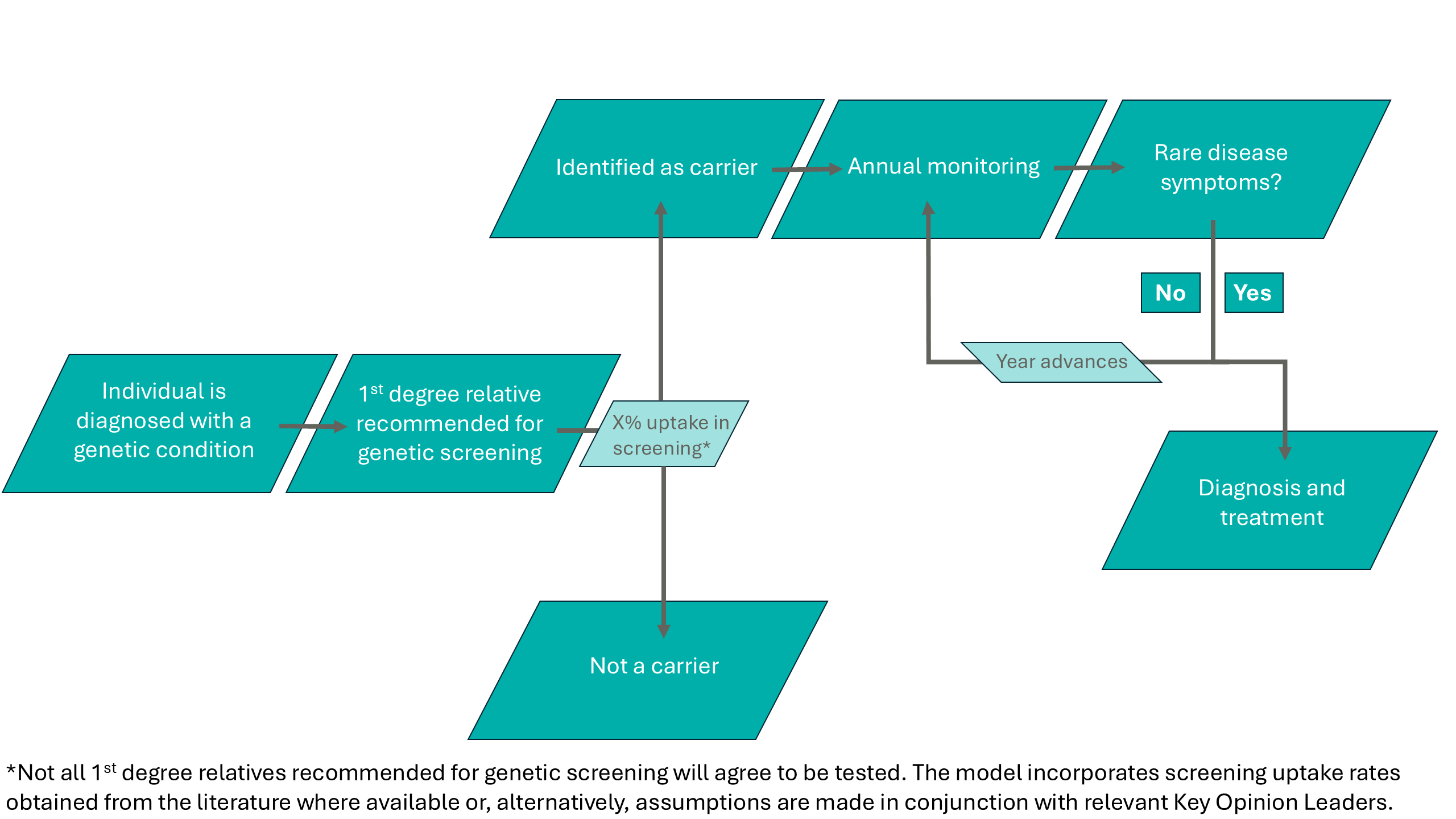
Figure 2: The cascade screening process assumed in the microsimulation model for first degree relatives of individuals diagnosed with a genetic condition.
The health and economic outcomes projected in the microsimulation model of the cascade intervention program are then compared to the outputs projected under a “no-change”, baseline scenario, enabling a direct comparison to be drawn between the two scenarios to quantify the potential impact of a cascade intervention.
Key stakeholders: Who needs to understand the potential impact of cascade interventions?
Quantifying the impact of earlier diagnosis through cascade screening provides powerful evidence for those working to improve outcomes for patients with genetic diseases, including:
Pharmaceutical and biotech companies – who can use this data to understand how a cascade intervention would lead to more genetic disease patients being diagnosed earlier, enabling them to gain access to the required therapies and treatment pathways.
Patient advocacy groups – who require data-driven evidence of the positive health and economic impacts that the introduction of a cascade screening program may have, in order to more effectively lobby policymakers and support the case for change.
Government and public health bodies – who need data on the health and economic benefits of cascade screening in order to justify resource allocation towards the implementation of a cascade screening intervention.
Healthcare organisations and providers – who need data on the impact of cascade screening to effectively plan for its implementation and the resource reallocation that may be required to meet the demand of newly diagnosed patients.
If you would like to further explore how evidence to support cascade screening programs can be generated, please contact us at info@healthlumen.com.