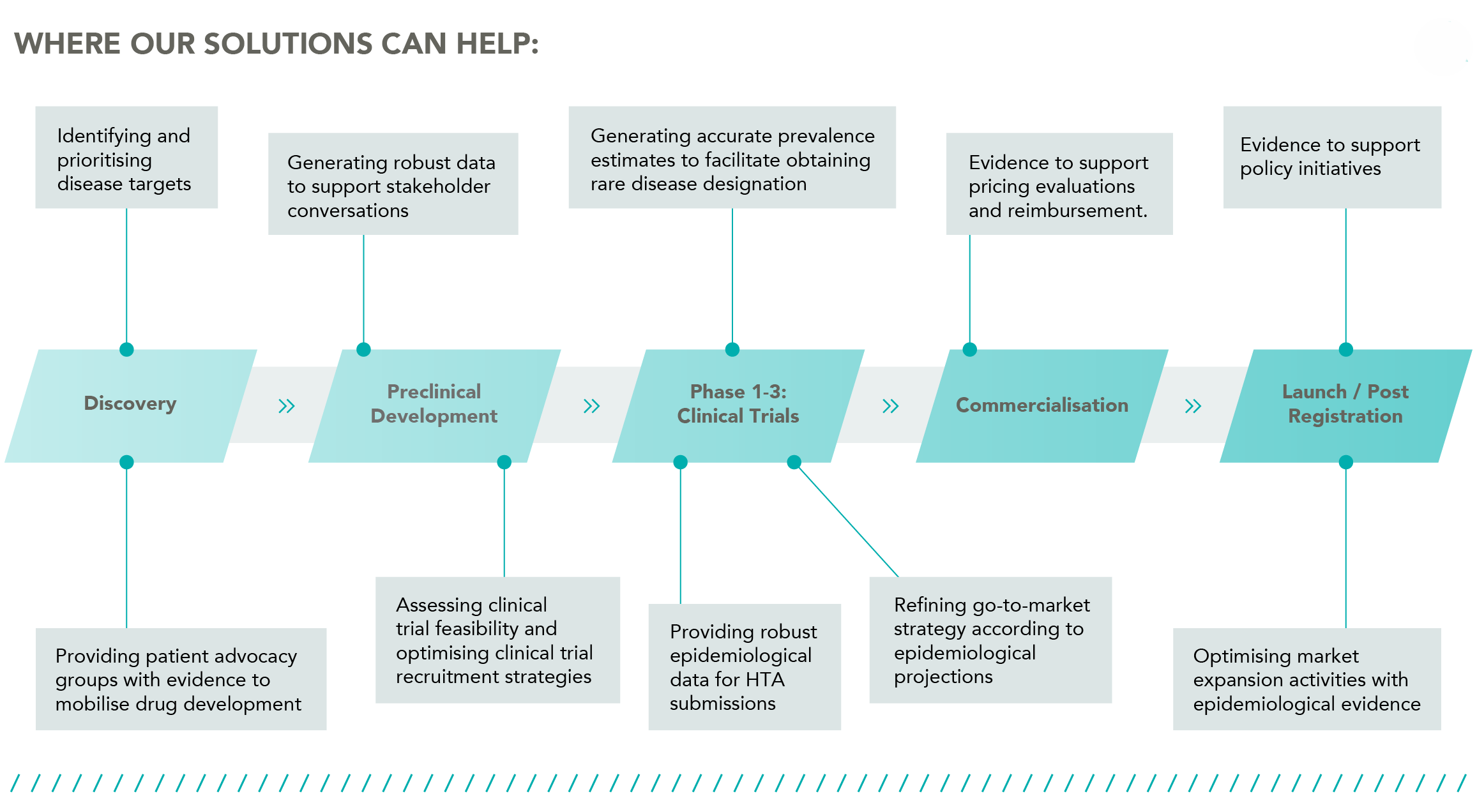
We combine our unique burden of disease modelling with genetic database analysis, literature reviews and policy analysis to help life science companies working on non-communicable diseases (NCDs) and rare genetic diseases at each stage of the drug development lifecycle, from R&D through to market access.
Our epidemiological modelling covers a wide range of scenarios for NCDs and rare genetic diseases:
Pharmaceutical and biotech companies, biotech investors, and public sector organisations involved in the following:
We have spent over a decade developing our microsimulation software to help you make the best decisions at each stage of the product development lifecycle.
We’ll help you define the population you need to look at, so you are confident you’re asking the right questions and are clear on what you need our answers to achieve.
We’ll create a starter scenario – inputting our robust patient population prevalence estimates – and project different “what-if” scenarios into the future. You can then compare the outcomes of different kinds of interventions to quantify their potential effectiveness.
We’ll interpret the data for you, giving you plain language answers to your original questions. That will help you translate those answers into reliable, data-driven decisions.